Selected Impacts
RESEARCH INTO POLICY
Dr. Nina Sayer’s JAMA Network Open article on impact of clinician burnout on PTSD psychotherapy efficacy featured in HSR Publication Brief for VA Central Office
This article reported results of a secondary data analysis from the SCORE PTSD study (IIR 17-178) that sought to identify sources of systematic variation between therapists in outcomes from evidence-based psychotherapies for PTSD. Researchers found that therapist burnout was prospectively associated with reduced effectiveness of guideline-recommended psychotherapies for PTSD. This finding underscores the effect of therapist-level factors in delivering trauma-focused therapies for PTSD. The publication brief was forwarded to VHA Central Office leadership. The article was also featured in four media articles and the VA National Center for PTSD’s Clinician’s Trauma Update publication (June 2024), which is disseminated to over 76,000 trauma clinicians.
Dr. Melissa Polusny was invited by resilience and military researchers at the University of Athens, Greece to provide a workshop to the Hellenic Armed Forces (Greek Pentagon)
Through a 17-year partnership with the Minnesota Army National Guard (MNARNG), Dr. Polusny has established a nationally recognized program of research in post-deployment mental health risk and resilience. As a result of her expertise, Dr. Polusny was invited to provide a workshop on conducting resilience research within the military context to the Hellenic Armed Forces (Nov. 2023). This workshop provided the Greek Pentagon with a framework for operationalizing resilience and generated discussion about key methodological considerations for designing future resilience research. Dr. Polusny has continued to collaborate with investigators from the National and Kapodistrian University of Athens and the Hellenic Armed Forces on a collaborative project to develop an instrument to assess resilience among peacekeeping forces and other military personnel. She also presented results of her ARMOR trial, a large multilevel, prospective longitudinal study investigating resilience processes that promote adaptation among National Guard service members. These findings are helping develop and disseminate resilience-building strategies for military personnel and Veterans.
Results of Dr. Diana Burgess’ Learning to Apply Mindfulness to Pain (LAMP) study published and shared with VHA national leaders
The LAMP study examined two scalable, telehealth mindfulness-based interventions (MBIs) versus usual care for Veterans with chronic pain. Both programs were found to improve pain outcomes and related conditions (e.g., depression, PTSD, etc). Dr. Burgess presented results at a Patient Care Services senior manager meeting and was invited to give presentations for NIH, the Interagency Pain Research Coordinating Committee (IPRCC), and the Whole Health VA ECHO webinar. The LAMP publication was also selected by Dr. Kilbourne to be featured in a HSR publication brief that was sent to VHA leaders (Aug. 2024). Additionally, it was featured in 24 news stories from 18 media outlets. Dr. Burgess disseminated findings via four podcasts/interviews (e.g., CBS radio, “The Mission Continues” podcast). This demonstrates impact within and outside VA.
Dr. Elisheva Danan’s groundbreaking VA HPV self-collection research is helping to reshape the approach to cervical cancer screening both within and outside VA
In FY24, she was invited to present this research to the Federal Cervical Cancer Collaborative Interagency Partners Team Monthly Meeting (Nov. 2023) and to the public through the Health Resources & Service Administration (HRSA) Office of Women’s Health webinar (Jan. 2024). The HRSA webinar had over 500 registered participants, and the recording was later posted on YouTube. Dr. Danan’s expertise in patient-centered cervical cancer screening was further sought as a subject matter expert in HRSA's CERV-Net: A Cervical Cancer ECHO Learning Series for Safety-Net Settings (March 2024). Dr. Danan was also invited to attend the 2024 American Cancer Society’s National Roundtable on Cervical Cancer HPV Self-Sampling Summit where she participated in small group discussions and brainstorming sessions related to the introduction of self-collected HPV testing in the US.
RESEARCH INTO PRACTICE
Dr. Princess Ackland was invited to film on Present-Centered Therapy (PCT) for the VA National Center for PTSD (NCPTSD) PTSD Treatment Decision Aid
The PTSD Treatment Decision Aid is one of the primary tools created by the National Center for PTSD to aid in the dissemination of evidence-based treatments for PTSD and support shared decision making. It includes videos recorded by clinicians describing the treatment options. Due to her nationally recognized expertise, Dr. Ackland was invited to film educational videos for the Decision Aid on Present-Centered Therapy (PCT), a therapy suggested by the VA/DoD PTSD clinical practice guidelines for the management of PTSD. Dr. Ackland was previously involved with two RCTs (CSR&D funded SPLE-003-11S and PCORI funded COMPASS trial) that have helped to establish the effectiveness of PCT and cement Dr. Ackland as one of only a few VA experts in the delivery of PCT. This Decision Aid is available on the VA National Center for PTSD’s website and serves as a resource for Veterans to learn about effective PTSD treatment options. The PCT videos are estimated to be available summer 2025.
Dr. Beth DeRonne developed and implemented resources to improve chronic pain treatment within Minneapolis VA
In collaboration with Dr. Kara Wong (Facility PMOP Coordinator), Dr. DeRonne implemented a non-pharmacological intervention for patients with chronic pain (“Empowered Relief”) within the Minneapolis VA. Monthly offerings of this 2-hour evidence-based intervention began in June 2024. In addition, Dr. DeRonne led the creation of a patient-facing document that lists all self-referral options available for patients with pain at the Minneapolis VA. This product consolidates numerous flyers/pamphlets into one document that is easier to share and discuss with patients. It will be broadly disseminated via Minneapolis VA Medical Media. These resources are increasing access to behavioral and self-management strategies for chronic pain and empowering patients to utilize them.
Dr. Anne Melzer’s strategic initiative demonstrates considerable impact in increased implementation of high-quality Lung Cancer Screening across VISN 23
This initiative aims to improve lung cancer screening for rural Veterans using centralized nurse coordination for community care. In the first year, the newly established Care in the Community ‘Referral Coordination Initiative’ processed over 3000 consults, recapturing 23% of patients from community care into VA, discharging ineligible patients (15%), enrolling 100% of community care patients in the VA’s centralized screening program, and increasing appropriate pulmonary consultation for suspicious lung nodules from 45% to 100%. At the second pilot site at the Fargo VA, over 1400 consults have been processed with 18% of care in the community eligible patients recaptured into the VA. These successful results have been shared with leaders from VISN 23 and the Lung Precision Oncology Program (LPOP) and will be presented at the upcoming LPOP conference. In FY24, Dr. Melzer also co-developed a toolkit for integrating tobacco treatment into lung cancer screening along with the Tobacco Use Treatment National Program Office that will go live Fall 2024.
INFOGRAPHICS
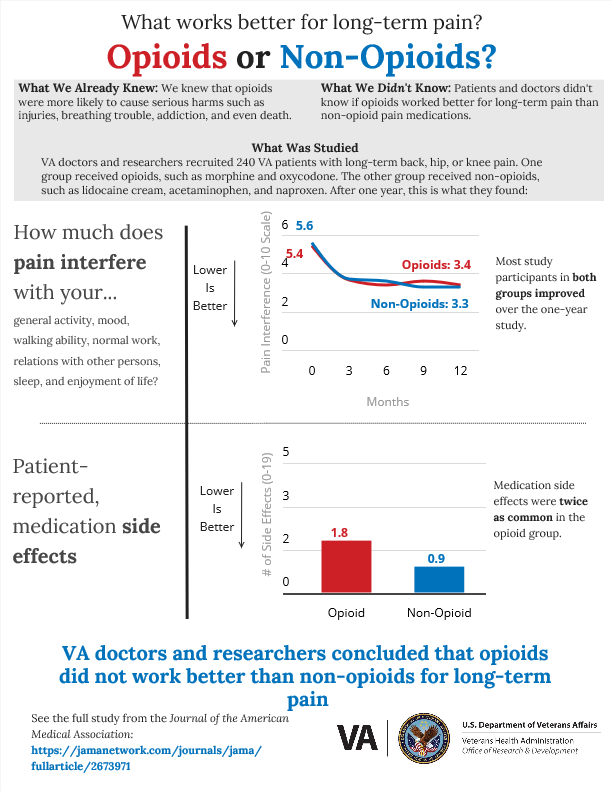 |
CCDOR researcher Dr. Erin Krebs and team worked with a panel of Veterans to develop an infographic to share results of the SPACE study with the community. Click the link below to view and share a copy of the infographic. https://infogram.com/opioids-vs-non-opioids-space-trial-va-research-1hke60d1drle25r |
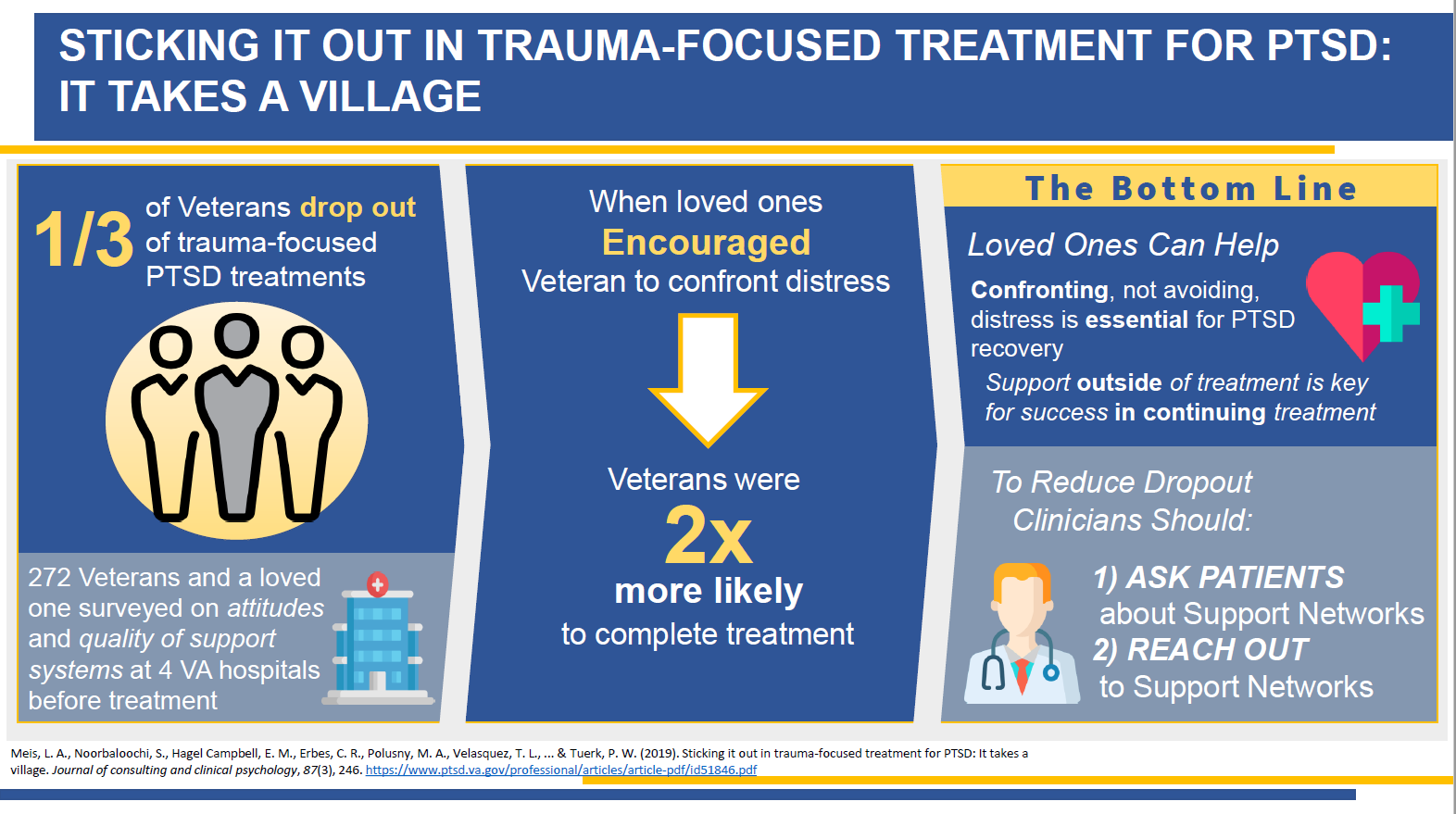 |
Dr. Laura Meis collaborated with CCDOR's Communication Committee to create a visual abstract of her article, "Sticking It Out in Trauma-Focused Treatment for PTSD: It Takes a Village," that was published in the Journal of Consulting and Clinical Psychology. View Visual Abstract |
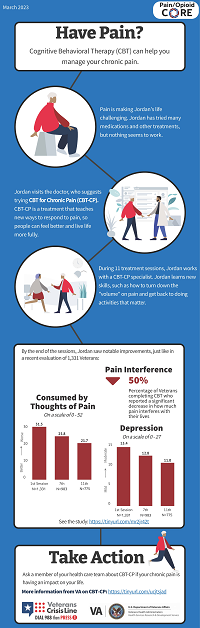 |
Dr. Tracy Sides and Dr. Erin Krebs, along with the Pain/Opioid Consortium of Research team, launched an initiative to produce a series of "infographics" which summarize results from a health services research study that is relevant to patients with chronic pain, opioid use, or opioid use disorder. Veteran input informs each stage of the production process. The infographic highlighted here shares information about Cognitive Behavioral Therapy for Chronic Pain in Veterans. Click the link below to view and share a copy of the infographic. All current Pain/Opioid CORE infographics are available on their website. |



















